Nitric oxide
Nitric oxide (NO), an endogenous diatomic free radical, mediates multiple physiological processes including angiogenesis, blood pressure regulation, wound healing, and the immune response. In vivo NO production is achieved by the three nitric oxide synthase (NOS) enzymes: endothelial, neuronal, and inducible NOS (eNOS, nNOS, and iNOS, respectively). The NOS enzymes generate NO at varied concentrations (nM–µM) and kinetics dependent on the enzyme location and purpose. For example, low concentrations of NO are generated from endothelial and neuronal NOS for blood vessel dilation/formation and various roles in neurotransmission. Activation of the inducible NOS isoform by immunological stimuli (e.g., lipopolysaccharides from bacteria) causes sustained NO release at high concentrations for eradicating foreign pathogens, which is central to the innate immune response. It is these critical physiological roles of endogenous NO that inspired us to design new NO-releasing macromolecular scaffolds and surfaces for use as antimicrobials and implanted device coatings. Along with this major research thrust, we also focus on developing NO-selective electrochemical sensors which are capable of measuring NO directly in complex environments (e.g., serum, wound fluid, blood).
Dendrimers
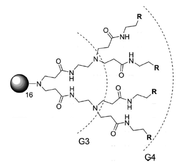
Dendrimers–hyperbranched polymeric macromolecules–are biocidal scaffolds with proven antibacterial activity that is dependent on the dendrimer generation (size) as well as the identity, charge, and hydrophobicity of the terminal functional groups. As the number of terminal groups predictably increases with dendrimer generation, the size and number of functionalized surface groups are easily tailored, making this class of molecules well-poised for investigating the relationships between antibacterial efficacy and surface modification. In addition, small dendrimer size results in their ability to associate with or cross bacterial membranes, providing an effective scaffold for the delivery of other antimicrobial agents. A current research focus in our group involves combining the contact-based, non-depleting antibacterial properties of dendrimers with the short-lived, release-based biocide nitric oxide. Functionalizing the dendrimer exterior prior to NO-loading has been our strategy for the development of multimodal combination therapeutics, which we anticipate will increase bactericidal efficacy against a wide range of bacterial strains. In particular, we are investigating the ability of NO-releasing dendrimers to eradicate bacteria that are common to dental caries (i.e., S. mutans) and periodontal disease (i.e., P. gingivalis, A. actinomycetemcomitans), as well as those bacteria and biofilms associated with chronic wounds (i.e., P. aeruginosa, S. aureus, MRSA).
Chitosan
In airway diseases associated with inflammation or chronic infections (e.g., asthma and COPD), exhaled NO concentrations are increased, especially during exacerbations. Surprisingly, exhaled NO levels in CF patients are similar to or decreased compared to healthy individuals, despite the intense inflammation associated with CF. While research into the causes and implications of low exhaled NO concentrations in CF patients is still ongoing, new therapeutic approaches are being designed to supplement NO levels in CF airways. To this end, the Schoenfisch lab has developed NO-releasing chitosan oligosaccharides. Chitosan oligosaccharides are particularly attractive as scaffold for pulmonary delivery due to their ability to be nebulized, mucoadhesive nature, low toxicity, and biodegradability. In order to store and controllably release NO, chitosan oliogosaccharides can be modified with either N-diazeniumdiolate or S-nitrosothiol moeities. Using N-diazeniumdiolate NO-donors, chitosan oligosaccharides are capable of releasing biocidal levels of NO. These scaffolds have demonstrated efficacy against P. aeruginosa biofilms–bacterial communities that are commonly implicated in chronic airway infections–at concentrations found to be non-toxic to mammalian cells.