Glucose Biosensors
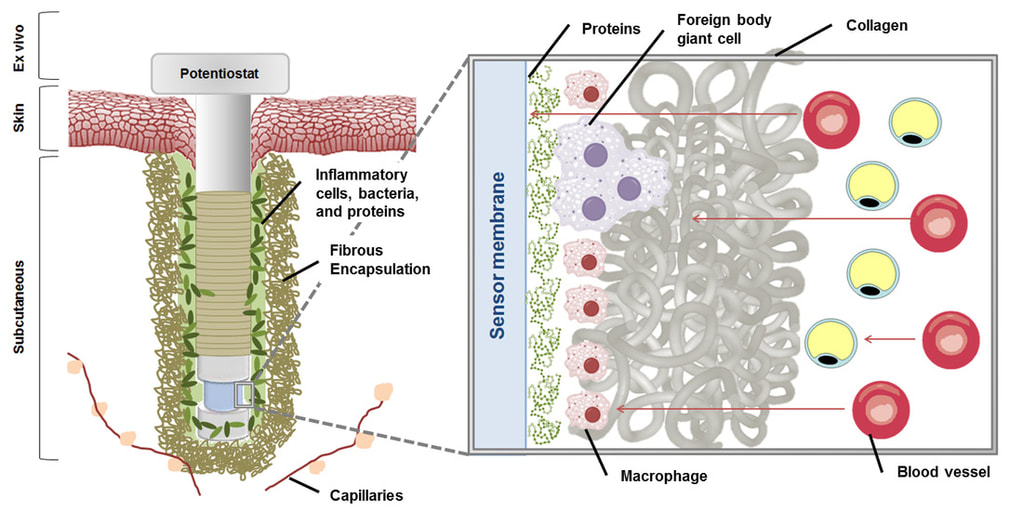
Implantable electrochemical glucose biosensors are currently used for home blood glucose measurement by diabetic patients. Despite the approval of these sensors for monitoring blood glucose by the U.S. Food and Drug Administration, an obstinate foreign body response (FBR) has been linked to their diminished and inadequate analytical performance. Typical characteristics of the FBR to all implanted devices include infiltration by inflammatory cells that consume glucose and release electroactive interfering chemical species, both of which negatively impact sensor performance. Eventually, the implant is isolated from native tissue by a thick collagen capsule generally results in device failure, as glucose diffusion to the sensing electrode is impeded.
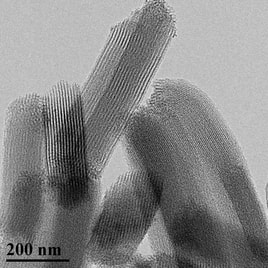
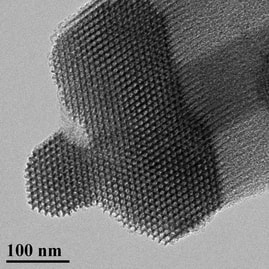
In order to minimize the FBR and improve the fate of implanted glucose biosensors, the Schoenfisch group has designed NO-releasing polymeric membranes that serve as the sensor’s outermost, tissue-contacting coating. To date, doping NO-releasing silica nanoparticles into biomedical grade polyurethanes has been our most successful strategy for achieving NO release without compromising sensor performance. Total NO storage and NO-release kinetics from the coatings can be controlled by changing the type of nanoparticle NO donor and the identity of the polyurethane coating. As a central theme in this research project, our group has carefully examined multiple aspects of the FBR with respect to NO-release kinetics from subcutaneous implants, including tissue inflammation, subcutaneous glucose transport, and actual in vivo sensor performance. We have determined that extended NO release (i.e., for several days) results in improved tissue characteristics and better sensor performance relative to both short-term (<1 day) and non-NO-releasing implants. Based on these observations, current research in our group focuses on developing new materials that release NO for even longer durations – this presents a great challenge, as most NO donors only release NO for <24 hours. Through a combination of innovative solid-state chemistry and stabilization of the NO donor, we have designed porous silica nanomaterials that can release NO for more than 7 days.
Through further analytical/materials work, research in the Schoenfisch group aims to evaluate how pore structure, ordering, and integrity of the porous silica impacts NO release. In the future, we intend to integrate these new materials with our polyurethane glucose sensor coatings. The ultimate goal of this project will be to evaluate the tissue response and actual sensor performance in a diabetic animal model in order to accurately recapitulate human physiology. Testing in diabetic animals will allow us to assess the potential benefits of NO to sensor performance under relevant tissue conditions of diabetes (e.g., impaired wound repair, restricted blood flow).